

Enzyme catalysis is an area of fundamental importance in different areas. This chapter offers a concise overview to the fundamental principles and mechanisms of action, catalysis inhibition and its pharmaceutical applications. Additionally, this section also covers basics information related with enzymes such as its structure, function and different properties.
This article is available under the terms of the IOP-Standard Books License
All rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the publisher, or as expressly permitted by law or under terms agreed with the appropriate rights organization. Multiple copying is permitted in accordance with the terms of licences issued by the Copyright Licensing Agency, the Copyright Clearance Centre and other reproduction rights organisations.
Permission to make use of IOP Publishing content other than as set out above may be sought at permissions@iop.org.
Saurabh Bhatia has asserted his right to be identified as the author of this work in accordance with sections 77 and 78 of the Copyright, Designs and Patents Act 1988.
The cell is the structural and functional unit of life—the basic building block of living systems. Cells have the capability to effectively utilize biocatalysts, known as enzymes, which have outstanding catalytic efficiency and both substrate and reaction specificity. Enzymes have amazing catalytic power and their high level of specificity for their substrate makes them suitable for biological reactions. They are crucial for cellular metabolism. Each and every chemical reaction that takes place in plants, micro-organisms and animals proceeds at a quantifiable rate as a direct result of enzymatic catalysis. Most of the history of biochemistry is directly or indirectly related to the history of enzyme research. Catalysis in biological systems was initially reported in the early 1800s based on research into the digestion of meat. In this report the catalytic activity of secretions from the stomach, the conversion of starch into sugar by saliva, and various plant extracts were reported.
In 1837, Berzelius documented the catalytic nature of fermentation. In the 1850s Louis Pasteur reported that fermentation was a process initiated by living organisms. During this study it was reported that the fermentation of sugar into alcohol by yeast was catalyzed by ferments. He also hypothesized that these ferments are close to the structure of yeast. These ferments were later called enzymes (in yeast). The key breakthrough in the history of enzymes came in 1897 when Edward Buchner isolated, from yeast cells, the soluble active form of the set of enzymes that catalyzes the fermentation of sugar to alcohol. Emul Fischer reported the first systematic studies on enzyme specificity in the early twentieth century [1]. Later, in 1926, James Sumner extracted urease in pure crystalline form from jack beans [2]. He also recognized the protein nature of urease. In 1930, John Northrop and his co-workers crystallized pepsin and trypsin and established them as proteins [3]. In subsequent years enzymology developed rapidly (table 1.1). The important developments during this period are: the elucidation of major metabolic pathways, such as the glycolysis and tricarboxylic acid cycle; the detection of numerous biochemical events of digestion, coagulation, muscular contraction and endocrine function, and their roles in the maintenance, control and integration of complex metabolic processes; the kinetic backgrounds to explain the observations of enzyme action and inhibition; and the development of protocols for examining the structures of functionally sensitive proteins. There has been exhaustive research on enzyme-catalyzed reactions and enzymes involved in cell metabolism. At present, 2000 different enzymes have been recognized, each of which catalyzes a different chemical reaction. Currently, more focus is being directed towards the application of enzymes. The high efficiency of enzymes makes them commercially valuable and their specificity of action is offering diverse advantages in clinical medicine.
Table 1.1. Chronology of enzyme studies.
| Name | Year | Work |
|---|---|---|
| Anfinsen | 1956–8 | The sequence of an amino acid regulates the folding pattern and activity of a ribonuclease. |
| Beatle and Tatum | 1940 | 'One gene one enzyme' hypothesis. |
| Bertrand | 1896–7 | Co-enzyme or co-ferment (currently known as co-factors). |
| Berzelius | 1835 | Concept of catalysis. |
| Berzelius | 1837 | Exploration of biological catalysis. |
| Briggs and Haldane | 1925 | Derivation of enzyme rate equations using the steady-state approximation. |
| Buchner | 1897 | Isolation of the soluble active form of enzymes from yeast cells. |
| Chances | 1943 | Application of spectroscopic techniques for studying enzymes. |
| Cori and Cori | 1937–9 | Muscle phosphorylase. |
| Duclaux Henri | 1898 | Nomenclature: substrate plus suffix 'ase'. |
| Fischer | 1894–5 | 'Lock and key' hypothesis of enzyme specificity. |
| Harden and Young | 1901–3 | Methods for the derivation of kinetic rate laws; principle of enzyme–substrate complex. 1906 Co-ezymase (NAD). |
| Jacob, Monod and Changeux | 1961 | Allosterism. |
| Koshland | 1953 | 'Induced fit' hypothesis. |
| Kuhne | 1878 | Explored trypsin catalyzed reactions; introduction of word 'enzyme'. |
| Michaelis and Menten | 1913 | Extension of the kinetic theory of enzyme catalysis. |
| Northrop and Kunitz | 1930–3 | Crystallization of proteolytic enzymes. |
| Pasteur | 1850 | Fermentation of sugar into alcohol by yeast. |
| Payen and Persoz | 1833 | Alcohol precipitation of thermolabile 'diastase' from malt. |
| Phillips, Johnson and North | 1965 | Three-dimensional structure of lysozyme obtained at 1.5 A resolution. |
| Sumner | 1926 | Crystallization of urease. |
| Sutherland | 1956 | Cyclic AMP adenyl cyclase. |
| Umbarger, Yates and Pardee | 1956 | Regulation of enzyme activity via feedback inhibition. |
| Wilhelmy | 1850 | Quantitative evaluation of the rates of sucrose inversion. |
1.2. Properties of enzymes
Enzymes are the complex protein molecules, often called biocatalysts, which are produced by living cells. They are highly specific both in the reactions that they catalyze and in their choice of reactants, which are known as substrates. An enzyme typically catalyzes a single chemical reaction or a set of closely related reactions [4]. Side reactions resulting in the wasteful formation of by-products are rare in enzyme-catalyzed reactions, in comparison to uncatalyzed ones. Enzymes can also be defined as soluble, colloidal and organic catalysts that are produced by living cells, but are capable of acting independently of the cells [4]. Enzymes are currently being used in diverse areas in the food, feed, paper, leather, agriculture and textiles industries, resulting in major cost reductions. Simultaneously, rapid scientific progress is now encouraging the chemistry and pharmacological industries to embrace enzyme technology, a trend supported by concerns regarding energy, raw materials, health and the environment. One of the most common advantages of enzymes is their ability to function continuously even after their removal or separation from the cells. This means that even after the separation of cells from in vivo environments, they continue to work efficiently under in vitro conditions; we can conclude that these biocatalysts remain in an active state even after their isolation. Principally, enzymes are non-toxic, biodegradable and can be produced in ample amounts by micro-organisms for industrial applications. In this chapter, the isolation, production, purification, utilization and application of enzymes (in soluble and immobilized or insoluble form) are discussed in detail. Procedures such as recombinant DNA technology and protein engineering are frequently used to produce more efficient and beneficial enzymes. The industrial production and utilization of enzymes is an important part of industry. Interdisciplinary collaboration between areas such as chemistry, process engineering, microbiology and biochemistry is required to develop the best possible enzyme technology, and eventually to achieve increased production and maintain the enzyme's physico-chemical properties under in vitro environments.
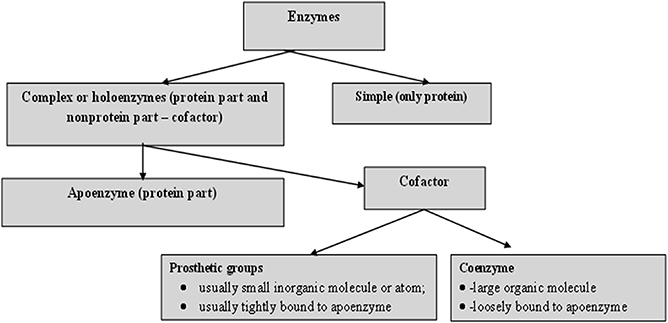
For catalytic action, small quantities of an enzyme are sufficient, where this quantity of enzyme is much smaller in comparison to its substrates. The overall concentration of substrate transformed per mass of enzyme is often very large. Without exception, all enzymes are proteinaceous and exhibit all the properties of a protein. The treatment of enzymes by extreme temperature or extreme pH, or by treatment with other denaturing agents, results in the complete loss of catalytic activity. Structural configurations such as the primary, secondary, tertiary and quaternary structures of enzyme proteins are essential for their catalytic activity. The degree of catalytic activity chiefly depends on the integrity of the enzyme's structure as a protein. As per reports, enzymes have molecular weights ranging from about 12 000 to over 1 million Da. A number of enzymes consist only of polypeptides and contain no chemical groups other than amino acid residues, e.g. pancreatic ribonuclease. Numerous enzymes require a specific, heat stable, low molecular weight organic molecule, known as a co-enzyme. Moreover, a number of enzymes require both a co-enzyme and one or more metal ions for activity. A complete biochemically active compound is formed by the combination of a catalytically active enzyme (also called the protein part) with a co-enzyme or a metal ion—this is called a holoenzyme. The protein part of a holoenzyme is called an apoenzyme. In this arrangement a co-enzyme may bind covalently or noncovalently to the apoenzyme. In certain enzymes the co-enzyme or metal ion is only loosely and transiently bound to the protein. However, in others it is tightly and permanently bound, in which case it is known as a prosthetic group. A prosthetic group signifies a covalently bound co-enzyme. According to reports, co-enzymes and metal ions are stable under heating, while the protein part of an enzyme (the apoenzyme), is denatured by heat.
Prosthetic groups may be classified functionally into two major classes: co-enzymes and co-factors. Co-enzymes may be considered to be biosynthetically related to the vitamins, such as the co-enzyme nicotinamide adenine dinucleotide (NAD) which is vital for cellular energy metabolism and integrates the vitamin niacin into its chemical makeup. Moreover, a co-enzyme may be considered as a co-substrate, experiencing a chemical transformation throughout the enzyme reaction (NAD is reduced to NADH), the reversal of which requires a separate enzyme, perhaps from a different cellular site. Co-enzymes might thus travel intra-cellularly between apo-enzymes and, by transferring chemical groupings, integrate several metabolic processes. Table 1.2 shows a list of the more common co-enzymes and their functions. In contrast to co-enzymes, co-factors, such as pyridoxal phosphate or hem groups, remain with one enzyme molecule and in conjunction complete a cycle of a chemical change brought about by one enzyme turnover [5]. Other enzymes, such as carboxypeptidase, require metal ions as co-factors, the divalent cations Mg 2+ , Zn 2+ and Mn 2+ being the most common; these are often called enzyme activators [6]. Table 1.3 lists several enzymes and their respective co-factors.
Table 1.2. Several co-enzymes employed in the transfer of specific atoms or functional groups.
| Co-enzyme | Entity transferred |
|---|---|
| Thyamin pyrophosphate | Aldehydes |
| Tetrahydrofolate | Other one carbon groups |
| Pyridoxal phosphate | Amino groups |
| Nicotinamide adenine dinucleotide | Hydrogen atoms (electrons) |
| Flavin adenine dinucleotide | Hydrogen atoms (electrons) |
| Co-enzyme A | Acyl groups |
| Biocytin | CO2 |
| 3'-deoxyadenorylcohalamine (co-enzyme B12) | H atoms and alkyl groups |
Table 1.3. Several enzymes and their co-factors.
| Enzyme | Co-factor(s) | Enzyme | Co-factor(s) |
|---|---|---|---|
| Pyruvate kinase | K + and Mg + | Urease | Ni ++ |
| Nitrate reductase | Mo | Peroxidase | Fe ++ or Fe +++ |
| Glucose 6-phosphatase | Hexokinase | Mg + | |
| DNA polymerase | Zn | Glutathione peroxidase | Se |
| Cytochrome oxidase | Cu** | Cytochrome oxidase | Fe ++ or Fe +++ |
| Carbonic anhydrase | Zn ++ | Catalase | Fe ++ or Fe +++ |
| Arginase | Mn | Alcohol dehydrogenase | Zn ++ |
The role of a catalyst is to increase the speed of a chemical reaction. When the rate of a chemical reaction is governed by a soluble catalyst, which may result in a further increase in the rate of chemical reaction, it is called homogeneous catalysis. In this case catalysis occurs in a solution. When the catalyst is in a separate phase from the reactants, or when catalysis occurs on a insoluble surface or an immobilized matrix, it is known as heterogeneous catalysis. Enzymes are also called biological catalysts. These biological catalysts generally have the properties of homogeneous catalysts, however, a number of enzymes present in membranes are insoluble, and thus are called heterogeneous catalysts. Enzyme specificity is the absolute specificity of protein catalysts to identify and bind to only one or a few molecules. In this process the enzyme carries a defined arrangement of atoms in their active site to bind with the substrate. This active site on the enzyme should have a shape that accurately matches the substrates. Thus specificity is achieved when an enzyme with an active site binds with the chemical reactants (the substrates) at their active sites via weak bond interactions. To undergo a chemical reaction, this active site carries certain residues that form a temporary bond with the chemical reactants, termed the binding site, whereas the catalytic site carries the residues that are responsible for catalysis. Specificity is achieved when a substrate binds to an enzyme that has a defined arrangement of atoms in the active site. An enzyme always catalyzes a single type of chemical reaction, which involves the formation and breakdown of covalent bonds. Since they are specific to one particular reaction, this feature of enzymes is called reaction specificity, also known as absolute reaction specificity, i.e. no by-products are formed.
1.4. The structure of enzymes
Enzymes always act as catalysts and small quantities compared to their substrate are required to considerably increase the rate of chemical reactions, wherein the enzymes themselves experience no overall change [7, 8]. In contrast to all true catalysts, an enzyme does not alter the ultimate equilibrium position of a reaction, which is thermodynamically determined, thus merely the rate of completion of equilibrium of a feasible reaction is augmented. In addition to catalytic properties, enzymes exhibit the physico-chemical behavior of proteins: their solubility, electrophoretic properties, electrolytic behaviors and chemical reactivity [7, 8]. The primary structural configuration and catalytic action of enzymes is determined by the linear chain of amino acid residues linked via peptide bonds, which constitute a protein molecule. Localized folding of the primary structure is called a secondary structure, whereas the complete folding of the molecule is known as a tertiary structure. In contrast to these structural configurations, a quaternary structure is the agglomeration of several folded chains. The structural features of enzymes are shown in figures 1.1 and 1.2. In contrast to traditional chemical catalysts, e.g. hydrogen ions, heavy metals or metal oxides, which are most effective in organic solvents, at very high temperatures or at extreme pH values, enzymes operate most efficiently under very mild conditions. When using enzymes, there are certain issues that require attention, such as deviation from homogeneous aqueous solutions, physiological pH and temperature, which can rapidly destroy enzyme activity. However, under normal conditions the increase in reaction rate is rarely matched by their non-protein counterparts.
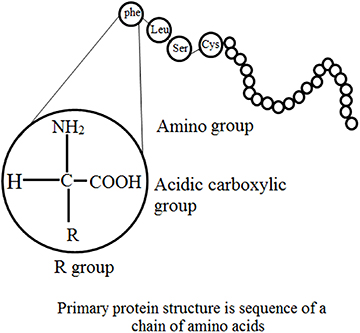
1.5. Structural features: primary and secondary structures
Three-dimensional analysis of the amino acid sequence of lysozyme of hen's egg white has demonstrated some features essential for primary structure [9, 10]. These are:
Molecules derived from a similar source have a similar order of amino acid residues and appear to be random with no obvious predictability.
Even though numerous enzymes are intramolecularly crosslinked via disulfide bridges of cysteine, no branching occurs.
Current databases suggest that a small number of amino acids are extra and most are 'functional', i.e. the majority of them co-operatively control the higher orders of structural organization and therefore the catalytic activity. When comparing the primary structures of enzymes performing similar functions, wide structural homologies are detected in their sequence, mainly in the patterns of their nonpolar residues. For example, pancreatic juice contains five inactive precursors (zymogens), namely chymotrypsinogen A, B and C, trypsinogen and proelastase; all of these are activated to the respective proteases by proteolytic cleavage [11].
1.6. The mechanism of action of enzymes
The mechanism of action is based on a chemical reaction, in which the enzyme binds to the substrate and finally forms an enzyme–substrate complex. This reaction take place in a relatively small area of the enzyme called the active or catalytic site. In other words, the mechanism of enzyme action is based on the nature of the enzyme–substrate interaction, which accounts for the reaction specificity of the biological catalysts. The active or catalytic site of an enzyme is constituted by several amino acids, located at some distance from each other in the peptide chain. These amino acids are brought close together by the folding resulting from the secondary and tertiary structure of the enzymes. Side chains of amino acid residues at the catalytic site provide groups for binding with specific groups of the substrate. Co-factors assist the catalysis. The substrate forms bonds with amino acid residues in the substrate binding domain of the active site. The binding induces a conformational reaction in the active site. During the reaction, the enzyme forms a transition-state complex. As the products of the reaction disassociate, the enzyme returns to the original state. Two different models postulated for the mechanism of enzyme action are given below.
This is a rigid model of the catalytic site, proposed by Emil Fischer in 1894 [12]. The model explains the interaction between a substrate and an enzyme in terms of a lock and key analogy. In this model, the catalytic site is presumed to be preshaped. The substrate fits as a key fits into a lock. The drawback of this model is the implied rigidity of the catalytic site. The model cannot explain changes in enzyme structure in the presence of allosteric modulators.
In contrast to the above method, this model suggests a flexible mode for the catalytic site. To overcome the problems of the lock and key model owing to the rigid catalytic site, Koshland [13–15] suggested an induced fit model in 1963. The important feature of this procedure is the flexibility of the active site. In the induced fit model, the substrate induces a conformational change in the active site of the enzyme so that the substrate fits into the active site in the most convenient way so as to promote the chemical reaction. This method suggests competitive inhibition, allosteric modulation and inactivation of enzymes on denaturation.
The Michaelis–Menten theory of enzyme action [16] offers the basis for most current research on the mechanism of enzyme action. This concept of the enzyme–substrate complex scheme assumes the combination of the enzyme and substrate in phase one (occasionally known as the transition phase) of the enzyme activity and liberation of the enzyme and the products of the catalysis in phase two of the reaction.
Covalent catalysis is evidenced in enzymes capable of forming covalent bonds between the substance and the catalytic group of the active site [17]. A number of enzymes react with their substrates to form very unstable, covalently joined enzyme–substrate complexes, which undergo further reaction to yield products much more readily than in an uncatalyzed reaction. Several of the enzymes that exhibit covalent catalytic behavior are listed in table 1.4.
Table 1.4. Various enzymes exhibiting covalent catalytic behavior.
| Enzyme | Reactive group | Typical covalent enzyme–substrate intermediate |
|---|---|---|
| Chymotrypsin, trypsin, thrombin, esterase | Serine | Acylserine |
| HO–CH2–CH– | ||
| Phosphoglucomutase, alkaline phosphatase | Serine | Phosphorylserine |
| HO–CH2–CH– | ||
| Glyceraldehyde-3-phosphate dehydrogenase papain | Cysteine | Acylcysteine |
| HS–CH2–CH– |
1.7. Catalysis via chymotrypsin
Hummel and Kalnitzky suggested an enzyme mechanism through the depiction of the sequential transition states experienced by the enzyme–substrate complex during catalysis [18]. Chymotrypsin is a digestive enzyme, responsible for proteolysis (breakdown of proteins and polypeptides) in the duodenum. Chymotrypsin favorably breaks peptide amide bonds (the carboxyl side of the amide bond is a large hydrophobic amino acid). These amino acids contain an aromatic ring in their side chain that fits into a 'hydrophobic pocket' of the enzyme. It is stimulated in the presence of trypsin. Trypsin and chymotrypsin are both serine proteases with high sequence and structural similarities, but with different substrate specificity [19, 20].
As discussed above, chymotrypsin is a protease enzyme that cuts on the C-terminal phenylalanine, tryptophan and tyrosine on peptide chains [21]. Additionally, it is more specific for aromatic amino acids because of its hydrophobic pocket. Comparable to other serine proteases, chymotrypsin also catalyzes the hydrolysis of certain esters [22]. The molecular events involved in catalysis are called intermediary enzymology. Chymotrypsin, a protease, favorably accelerates breakdown of peptide bonds in which the aromatic amino acid (Phy, Try, or Trp) or bulky nonpolar R group (Met) contribute a carboxyl group. The synthetic substrate p-nitrophenyl acetate allows colorimetric analysis of chymotrypsin activity, as hydrolysis to p-nitrophenol, which is alkali, changes into the chromophore anionic forms.
The kinetics of chymotrypsin of p-nitrophenyl acetate can be considered in a 'stop-flow' apparatus. This procedure utilizes substrate quantities of enzymes and measures the events in the first few milliseconds [23]. The use of p-nitrophenyl acetate as a substrate offers the prospect of investigating solvent effects on both the acylation of the enzyme and the hydrolysis (deacylation) of the acyl enzyme [23]. The significant features of the slow-flow kinetics of chymotrypsin are:
- a burst phase featuring rapid liberation of an anion. - a subsequent 'steady-state' phase, with slower release of extra anion.In catalysis by chymotrypsin, the slow stage is hydrolysis of the chymotrypsin–acetate (CT–Ac) complex. When all the existing chymotrypsin has been converted to CT–Ac, no further release of p-nitrophenyl acetate anion can take place until more free chymotrypsin is released by the slow, hydrolytic elimination of acetate anion from the CT–Ac complex [24, 25]. The free chymotrypsin then is presented for further formation of chymotrypsin–p-nitrophenyl acetate complexes (CT–PNP) and CT–Ac complexes with attendant liberation of PNP. The development and decay of the enzyme–substrate complex, based on the Michaelis–Menten kinetics can be represented as [24, 25]:
where CT = chymotrypsin, PNP = p-nitrophenyl acetate, CT–PNP = chymotrypsin–p-nitrophenyl acetate complex and CT–Ac = chymotrypsin–acetate complex. In comparison to the hydrolysis of the CT–Ac complex, the formation of CT–PNP and CT–Ac complexs is relatively fast.
A 'charge relay network' acts as a proton shuttle during catalysis by chymotrypsin. The charge relay network of chymotrypsin encompasses three aminoacyl residues that are far apart in a primary structural sense, but close together in a tertiary structural sense. While most of the charged residues of chymotrypsin are present at the surface of the molecule, those of the charge relay network are hidden in the otherwise nonpolar inner side of the protein. These charges transmit residues which activate sequential proton shifts that shuttle protons in the opposite direction. An equivalent series of proton shifts is assumed to accompany the hydrolysis of the physiologic chymotrypsin substrate, e.g. a peptide.
Various enzymes, hormones and other physiologically active proteins are produced as inactive precursors (zymogens) that are further transformed to the active form by selective enzymatic cleavage (limited proteolysis) of peptide bonds. The final step to activating enzymatic function is limited proteolysis, either in a single activation step or in a consecutive series (cascade). The specificity of each activation reaction is evaluated by the complementarity of the zymogen substrate and the active site of the attacking protease. The arrangement of successive activation reactions is controlled by the specificity of each enzyme, while the extent of amplification of the initial stimulus is evaluated by the effectiveness of each activating step. Zymogen activation produces a prompt and irreversible response to a physiological stimulus, and is capable of initiating new physiological functions. Classical examples are the processes of hormone production, fibrinolysis, complement activation, blood coagulation, supra-molecular assembly, metamorphosis, fertilization and digestion. The zymogens of the pancreatic serine proteases, in particular, have functioned as models for detailed studies of the nature of the molecular changes that are involved in the intense increase in enzymatic activity that results upon incomplete proteolysis of the zymogen.
Specific proteolysis is a common means of activating enzymes and other proteins in biological systems. A number of proteins are manufactured and released in the form of inactive precursor proteins called proproteins. Various enzymes attain full enzymatic activity as they suddenly fold into their characteristic three-dimensional forms. In contrast, other enzymes are produced as inactive precursors that are successively activated by breakdown of one or a few specific peptide bonds. The inactive precursor is known as a zymogen (or a pro-enzyme). In other words, when the proteins are enzymes, the proteins are called pro-ezymes or zymogens (table 1.5). An energy source (ATP) is not required for cleavage [11]. Thus, in comparison to reversible regulation by phosphorylation, even proteins sited outside cells can be triggered by this means. An additional noteworthy difference is that proteolytic activation, in comparison with allosteric control and reversible covalent modification, occurs just once in the life of an enzyme molecule. Transformation of a proprotein to the mature protein includes selective proteolysis. This transforms the proproteins by one or more consecutive proteolytic clips to a arrangement in which the individual activity of the mature protein (its enzymatic activity) is expressed, e.g. the hormone insulin (proinsulin), the digestive enzyme chymotrypsin (chymotrypsinogen), a number of factors for blood clotting and for the blood clot dissolution cascades, and the connective tissue protein collagen (procollagen). Chymotrypsinogen consists of 245 amino acid residues, and is practically devoid of enzymatic activity. As the reaction starts, it is converted into a fully active enzyme. This occurs when the peptide bond joining arginine 15 and isoleucine 16 is cleaved by trypsin. The subsequent active enzyme, known as π-chymotrypsin, then acts on other π-chymotrypsin molecules. Two dipeptides are eliminated to form α-chymotrypsin (the stable form of the enzyme) [11]. The three subsequent chains in α-chymotrypsin remain interconnected to each another by two interchain disulfide bonds. The outstanding feature of this process is that cleavage of a single specific peptide bond alters the protein from a catalytically inactive form into one that is fully active. The transformation of prochymotrypsin (Pro-CT), a 2,4,5-aminoacyl residue polypeptide, to the active enzyme α-chymotrypsin includes three proteolytic clips and the formation of an active intermediate called π-chymotrypsin (π-CT) and consequently to the mature catalytically active enzyme α-chymotrypsin (α-CT). Examples of gastric and pancreatic zymogens are listed in table 1.5.
Table 1.5. Gastric and pancreatic zymogens.
| Active enzyme | Zymogen | Site of production |
|---|---|---|
| Chymotrypsin | Chymotrypsinogen | Pancreas |
| Trypsin | Trypsinogen | Pancreas |
| Carboxypeptidase | Procarboxypeptidase | Pancreas |
| Elastase | Proelastase | Pancreas |
| Pepsin | Pepsinogen | Stomach |
Generally, enzyme kinetics is defined as the study of the rate of reactions, i.e., how the substrate concentration impacts the velocity of the reaction. Enzyme kinetics involves optimization of bio-catalytic reactions to allow process design and scaling up processes to further increase the production and minimize the overall overhead costs of various procedures. Kinetic investigations in the branch of biochemistry concerned with enzymes can be categorized into three types:
Transient-state kinetics: This is the stage of reaction before the steady or rapid-equilibrium state, and involves quick reactions between the enzymes and substrate. These sudden changes in the reaction mixture when the substrate and enzymes are mixed require advance equipment to monitor the reaction before it changes into the steady state. The mechanisms of the reaction are associated with the enzyme structural configuration. Basic steps are involved during an enzyme-catalyzed reaction, which allow the direct study of the intermediates and products formed during a single enzyme cycle, which may further help in direct analysis of individual reaction steps for short times. In this type of reaction a sufficient concentration of enzymes is used to witness the intermediate and product formation.
Steady-state kinetics: This is the phase in which the rate of formation of intermediates and the rate of decomposition remain the same, and thus the concentrations of reactive intermediates remain the same. During this reaction substrate concentration is greater than enzyme concentration. The Michaelis–Menten enzyme kinetic (figure 1.3) can be considered as the most often studied reaction for several enzymes. For example, chymotrypsin (protease) with a high concentration of substrate achieves maximum velocity of the reaction (called the first order of reaction) but at a certain point the substrate occupies all binding sites of the enzyme, after which further addition of substrate does not increase the rate. This is called the zeroth order of reaction (the steady state). It is the phase in which the enzyme and substrate concentrations cannot be determined using the dissociation constant. Thus steady-state enzyme kinetics is based on the theory that a catalytic reaction remains constant if the reaction is not exposed to continuous changes.
Rapid-equilibrium kinetics: This the phase in which both the enzyme and substrate concentrations can be determined using the dissociation constant. During this procedure total enzyme concentration remains constant during the reaction and the concentration is very small compared to the amount of substrate. In this reaction, before the rate-determining reaction, the reactions are in equilibrium with their components, thus this stage is called rapid-equilibrium kinetics.
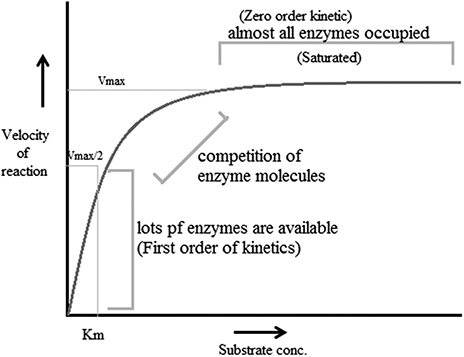
According to reports, factors that affect enzyme-catalyzed reactions also affect the velocity of a reaction. These factors are called modifiers of enzyme-catalyzed reactions. These modifiers can be divided into two classes: inorganic modifiers (enzyme activators) and organic modifiers (enzyme inhibitors). These factors can have different types of effects on the velocity of the reaction; nevertheless the most vital effect is that they offer many pathways to products, e.g. when one modifier is bound to an enzyme, it alters the rate of reaction and thus forms two rate constants. However, when two modifiers participate, there are five self-regulating equilibria, resulting in three paths for making products.
There are two mechanisms, single-substrate and multiple-substrate, that are helpful in studying the different stages of enzymatic reactions. Understanding these stages helps in understanding the properties of enzymes. Certain enzymes have single substrates (a single substrate binding site), e.g. triosephosphate isomerase, whereas certain enzymes have multiple substrates molecules (multiple binding sites), such as dihydrofolate reductase, and bind with multiple substrates. After the exploration of specific RNA sequences required for RNA replication, new biocatalysts in the form of ribozymes have emerged with the potential to catalyze specific biochemical reactions. There is a misconception about biological catalysts that all biological catalysts are made up of proteins, which is not true; some are RNA-based catalysts (ribozymes and ribosomes). Both are important for many cellular functions. A major difference between enzymes and ribozymes is that RNA-based catalysts are restricted to only a few reactions; however, their reaction mechanisms and kinetics can be studied and classified by similar procedures. Enzyme-based mutation, in particular site-directed mutagenesis, is an important approach to alter genes and investigate the functional and structural features of enzymes, e.g. mutation of the enzyme present in Coprinus cinereus peroxidase offers an understanding of its increased thermostability. Challenges involved in studying cascades of reactions catalyzed by a multi-enzyme, e.g. proteasome involved in the ubiquitin–proteasome pathway, can be overcome by establishing understanding of the complex structure and the respective biochemical reactions. This understanding allows exploration of active sites, intermediate compounds, final products and their interrelation with complex machinery, as well as biochemical reactions. It has been well understood that enzymes that accelerate complex reactions have numerous substrates and involve complex enzyme kinetic mechanisms. As discussed above, most of the biochemical reactions occurring in the body are multi-substrate reactions. In such reactions two substrates are involved and yield two products (figure 1.4). These types of reactions involve the transfer of a compound from one compoment to another, e.g. when glucose reacts with ATP in the presence of hexokinase it forms glucose 6-phophaste and ADP. Here, phosphate from ATP is transfered to glucose to form glucose 6 phosphate. The mechanism of catalysis involves two types of reactions: sequential and non-sequential reactions. Sequential reaction results in the formation of a ternary complex. This means that both of the substrates involved in the reaction bind with an enzyme to form the product (figure 1.4). Sequential reaction is further divided into two types: the random and compulsory order mechanisms. As the name suggests, in a 'random' mechanism, either substrate can bind first and any product can leave first. In contrast to the random order mechanism, in the compulsory order mechanism the order of binding of the substrate and order of release of the product is specific; this is also called the Theorell–Chance mechanism (figure 1.4). In a non-sequential reaction, also called the 'ping-pong' mechanism, formation of ternary complex does not take place. In these types of reactions, when the first substrate binds with enzyme its product is released, and then the second substrate binds and its product is released. Such a reaction is called a double placement reaction. Thus only a single substrate binds at a time; this may be due to the presence of a single binding site on the enzyme. Major differences between the sequential and non-sequential reactions are that the formation of a ternary complex takes place only in the sequential reaction, and that in the sequential reaction both substrates bind to the enzyme and release products, while in the non-sequential mechanism the substrates bind and release their products one after the other (figure 1.4).
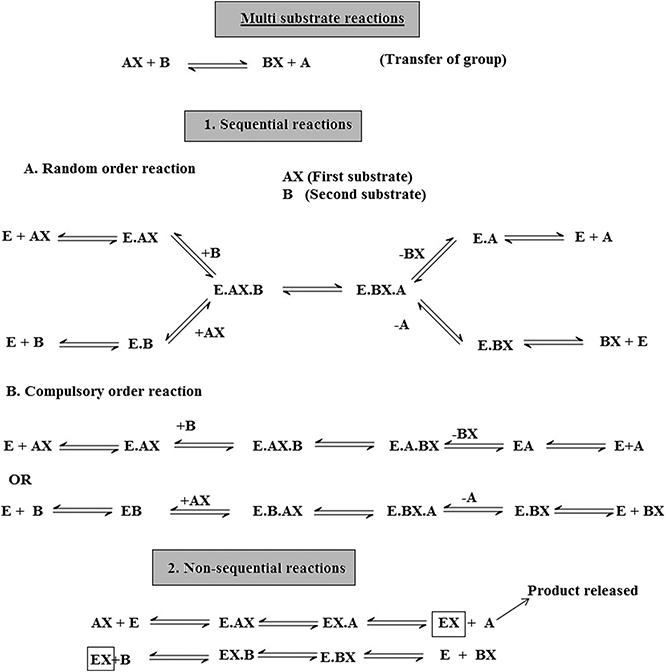
Another type of sequential mechanism is the systematic mechanism, which involves the addition of substrates and formation of products in a specific order.
Several protein enzymes use general acid–base catalysis as a way to increase reaction rates [26]. The amino acid histidine is optimized for this function because it has a pK(a) (where K(a) is the acid dissociation constant) near physiological pH [26].
When the substrate has been bound at the catalytic site, the charged functional groups of the side chains of neighboring aminoacyl residues may contribute in catalysis by behaving as acidic or basic catalysts. There are two extensive groups of acid–base catalysis by enzymes: general and specific (acid or base) catalysis. Specific acid or specific base catalysis are those reactions in which the reaction rates fluctuate under the influence of changes in H + or H3O + concentration, but are independent of the concentrations of the other acids or bases present in the solution. In contrast to specific catalysis, general acid or general base catalysis are the reactions whose rates are very reactive to all acids (proton donors) or bases (proton acceptors) present in the solution. To examine whether a given enzyme-catalyzed reaction is a general or specific acid or base catalysis, the rate of reaction is determined under two sets of circumstances:
at different pH values at a constant buffer concentration, andat constant pH values but at different buffer concentrations. Against this background, if the degree of the reaction deviates as a function of pH at a constant buffer concentration, the reaction is specific base/acid catalyzed if the pH is above/below 7.0. If the reaction rate at a constant pH rises as the buffer concentration increases, the reaction is general base/acid catalysis, if the pH is above/below 7.0.
Almost 25% of all enzymes include tightly bound metal ions or need them for activity. The major role of these metal ions is investigated using techniques such as x-ray crystallography, magnetic resonance imaging (MRI) and electron spin resonance (ESR). A metalloprotein is a protein that contains a metal ion co-factor. Metallozymes contain a certain amount of functional metal ion that is retained during the course of purification [27]. A metal-activated enzyme binds with metals less firmly, but needs to be activated by addition of metals. Four types of complexes are possible for the tertiary complexes of the catalytic site (Enz), a metal ion (M) and substrate (S) that exhibit 1:1:1 stoichiometry:
All of these complexes are possible for metal-activated enzymes. Metallozymes cannot form the EnzSM complex (substrate–bridge complexes), as the purified enzyme exists as Enz–M. Three generalization can be made:
The majority of the kinases (ATP: phosphotransferases) form substrate–bridge complexes of the type enzyme–nucleotide–M.
Phosphotransferases (phosphoenolpyruvate or pyruvate used as the substrate), enzymes catalyzing other reactions of phosphoenolpyruvate and carboxylases, form metal bridge complexes (Enz–M–S).
A particular enzyme may form one type of bridge complex with one substrate and a different type with another.
The metal ions participate in each of the four mechanisms by which the enzymes are known to accelerate the rates of chemical reaction:
Approximation of reactants. Covalent catalysis. General acid–base catalysis. Induction of strain in the enzyme or substrate.Metal ions are electrophiles (attracted to electrons) and share an electron pair forming a sigma bond. They may also be considered as super acids as they exist in neutral solutions, frequently having a positive charge which is greater than their quantity. Mn 2+ , Ca 2+ and Mg 2+ are the metal ions that are most commonly used in enzymatic catalysis. Two metal ions, iron and manganese are used in the form of haemprotein. Metal ions have the potential to accept electrons via sigma or pi bonds to successively activate electrophiles or nucleophiles. By means of donating electrons, metals can activate nucleophiles or act as nucleophiles themselves. The co-ordination sphere of a metal may bring together the enzyme and substrate or form chelate-producing distortion in either the enzyme or substrate [28]. A metal ion may also mask a nucleophile and thus avoid an otherwise probable side reaction. Metals can also function as three-dimensional templates for the co-ordination of basic groups on the enzyme or substrate.
1.8. Enzyme inhibition
Enzyme inhibition decreases the activity of an enzyme without significantly disrupting its three-dimensional macromolecular structure. Inhibition is therefore distinct from denaturation and is the result of a specific action by a reagent directed or transmitted to the active site region. When low molecular weight compounds interfere with the activity of enzymes by partially reducing or completely inhibiting the enzyme activity either reversibly or irreversibly, it is known as enzyme inhibition. The compounds responsible for such inhibition are called enzyme inhibitors. To protect the enzyme catalytic site from any change, a ligand binds with a critical side chain in the enzyme. Chemical modification can be performed to test the inhibitor for any drug value. Studies of enzymes can yield much information about the following:
A number of drugs useful in medicine, which seem to function because they can inhibit certain enzymes in malfunctioning cells.
The convenience of elucidating metabolic pathways in cells. The mechanism of the catalytic activity. The nature of the functional group at the active site. The substrate specificity of the enzyme.The pharmacological action of drugs is mainly based on enzyme inhibition, e.g. sulfonamides and other antibiotics. In the majority of cases the enzyme inhibited is known. The development of nerve gases, insecticides and herbicides is based on enzyme inhibition studies. There are two major types of enzyme inhibition: reversible and irreversible.
Reversible inhibitors efficiently bind to enzymes by forming weak non-covalent interactions, e.g. ionic bonds, hydrophobic interactions and hydrogen bonds. Reversible inhibitors do not form any strong chemical bonds or reactions with the enzyme, they are formed quickly and can easily be removed, in contrast to irreversible inhibitors. Reversible inhibition includes competitive inhibition, uncompetitive inhibition and noncompetitive inhibition. Irreversible inhibition includes group specific inhibition (reacts only to a certain chemical group), reactive substrate analogs (affinity label) and inhibitors that are structurally similar to the substrate and will bind to the active site, and mechanism-based inhibitors (enzymes transform the inhibitor into a reactive form within the active site).
1.9. Pharmaceutical applications
Currently, enzymes are often utilized for a broad range of applications such as: washing powders (e.g. proteases, lipases, amylases); textile manufacture (amylases and catalase to remove the starch); the leather industry (proteases to hydrolyze proteins); the paper industry; improvement of the environment; food production (enzyme-modified cheese/butter), processing (glucose oxidase for dough strengthening) and preservation; and medical applications. According to current reports, several enzymes are produced industrially and there are significant applications in the food industry (45% of use), detergent industry (35%), textiles industry (10%) and leather industry (3%). Details on the applications of individual enzymes are provided in table 1.6.
Table 1.6. Industrially produced enzymes from plant sources and their applications.
| Enzyme | Source(s) | Application(s) |
|---|---|---|
| β-amylase | Barley, soy bean | Baking, preparation of maltose syrup |
| Bromelain | Pineapple | Baking |
| Esterase | Wheat | Ester hydrolysis |
| Ficin | Fig meat | Tenderizer |
| Papain | Papaya | Meat tenderizer, tanning, baking |
| Peroxidase | Horse radish | Diagnostic |
| Urease | Jack bean | Diagnostic |
Enzymes have been used widely in diagnostic applications varying from immunoassays to biosensors. Enzyme immunoassay methods hold great promise for application under a wide variety of conditions. Under laboratory conditions they can be as sensitive as radio-immunoassays, but they can also be adapted as simple field screening procedures [29, 30]. The examination of enzyme quantity in the extracellular body fluids (blood plasma and serum, urine, digestive juices, amniotic fluid and cerebrospinal fluid) are vital aids to the clinical diagnosis and management of disease. Most enzyme-catalyzed reactions occur within living cells, however, when an energy imbalance occurs in the cells because of exposure to infective agents, bacterial toxins, etc, enzymes 'leak' through the membranes into the circulatory system. This causes their fluid level to be raised above the normal cell level. Estimation of the type, extent and duration of these raised enzyme activities can then furnish information on the identity of the damaged cell and indicate the extent of injury. Enzyme assays can make an important contribution to the diagnosis of diseases, as a minute change in enzyme concentration can easily be measured. Determination of the changes in enzyme level thus offers a greater degree of organ and disease differentiation in comparison to other possible clinico-chemical parameters, e.g. albumin or gamma globulin. Currently, the diagnostic specificity of enzyme tests is such that they are limited primarily to confirming diagnosis, offering data to be weighed alonside other clinical reports, owing to lack of disease specific enzymes. Table 1.7 includes a number of diagnostically important enzymes which are most often examined in clinic laboratories [29, 30].
Table 1.7. Diagnostically significant enzymes.
| Enzyme and abbreviation | Tissue source 1 | Reaction |
|---|---|---|
| γ-glutamyl transferase GGT | K L | γ-glutamyl peptide to γ-glutamylamino acid |
| Ornithine carbamoyltransferase | L | Carbamoyl-P to citrulline |
| OCT triacylglycerol lipase | Pa | Triacylglycerol to diacylglycerol and fatty acid |
| Lactate dehydrogenase LD | H L M K | Lactate to pyruvate |
| Isocitrate dehydrogenase ICD | L | Isocitrate to oxoglutarate |
| Hydroxybutyrate dehydrogenase HBD (LD I) | H | 2-hydroxybutyrate to 2-oxybutyrate |
| Fructose-biphosphate aldolase ALD | M H | Fructose-1,6-biphosphate to triosephosphate |
| Creatine lipase CPK | M H B | Creatine to creatine phosphate |
| Chymotrypsin CT | Pa | Proteins to polypeptides |
| Cholinesterase CHE | L | Acylcholine to fatty acid and choline |
| Aspartate aminotransferase GOT (AST) | H L M K B | Aspartate to glutamate |
| Alkaline phosphatase AP | B I L Pl K | Phosphate monoester to alcohol and Pi (pH 8–10) |
| Alanine aminotransferase GPT (AAT) | L | Alanine to gultamate |
| Acid phosphatase SP | Pr E | Phosphate monoester to alcohol and Pi (pH 8–10) |
| Acetylcholinesterase ACHE | B E | Acetylcholine to acetate and choline |
| α-amylase | Pa S | Starch to maltose |
| 5'-nucleosidase 5.N | Ht Pa | 5'-ribonucleotide to ribonucleoside |
1 B, brain; E, erythrocytes; H, heart muscle; Ht, hepatobiliary tract; I, intestinal mucosa; K, kidney; L, M, skeletal muscle; Pa, pancreas; P1, placenta; Pr, prostate gland; S, saliva.
The diseases of the liver and gastrointestinal tract were among the first to which serum enzyme tests were applied. They have proved to be most effective owing to the large size of the organs and the wide range and abundance of enzymes [32–36]. The liver-based enzymes GOT, GPT and AP are examined to evaluate the site and nature of liver disease. LD, GGT, OCT and CHE are also examined. Several enzymes employed in the diagnosis of liver diseases along with their respective levels are listed in table 1.8.
Table 1.8. Liver diseases and enzymes used in diagnosis [32–36].
| Disease | Enzyme used | Enzyme level |
|---|---|---|
| Solvent poisoning of liver | GOT, GPT and LD | GOT:GPT:LD 6500:3000:10 000 (U mI −1 ) |
| Hepatobiliary disease (obstructive jaundice) | GOT and GPT | 5–10 times normal level |
| Fatty liver | GPT | 2 times normal level |
| Chronic hepatitis and cirrhosis | All liver transaminases | 3–12 times normal level and inflammation of the liver |
| Acute hepatitis | GOT and GPT | 20–50 times normal level |
According to previous reports, no single enzyme has yet been reported to cure myocardial damage. The discovery of serum glutamine oxalacetic acid transaminase determination (GOT) in 1954 was considered a significant step forward in the diagnosis of acute myocardial infarction. A mixture of results from assays of CPK (creatine phosphokinase), HBD (α-hydroxybutyrate dehydrogenase) and GOT (glutamine oxalacetic acid transaminase)—each of which has been shown to be elevated in more than 90% of cases—is used for diagnostic purposes [37–39]. The level of CPK starts rising three to four hours after the initial onset of pain, followed in order by GOT and AST (HBD) which appear after approximately eight hours. The maximum levels are reached in the same sequence, CPK after 24 h, LD 1 after 36 h and AST after about two days. The rise in enzyme levels is fairly moderate, AST and CPK increase by four to ten times their respective normal levels and LD 1 is approximately five-fold higher than normal. An enzyme known as hyaluronidase (hyaluronate hydrolysis) has been reported to cure heart attack [38]. The activity of many enzymes including aldolase, malic dehydrogenase, isomerase and ICD may increase following myocardial infarction [38].
Skeletal muscle disorders include diseases of the muscle fibers (myopathies) or of the muscle nerves (neurogenic disorders) [40]. In myopathies CPJ, LD, ALD, GOT and GPT levels are raised. In the case of neurogenic diseases and hereditary diseases, CPK is occasionally raised (2–3 fold) [40]. Damage to the muscle may be due to extensive muscular exercise, drugs, physical trauma, inflammatory diseases, microbial infection or metabolic dysfunction, or it may be genetically predisposed. In muscular disorders the level of CPK is elevated in serum with the highest frequency and is assayed in the diagnosis of these disorders. An additional useful assayed enzyme is acetyl cholinesterase (AChE), which is significant in regulating certain nerve impulses [41]. Various pesticides affect this enzyme, so farm labors are frequently tested to be sure that they have not received accidental exposure to significant agricultural toxins. There are number of enzymes that are characteristically used in the clinical laboratory to diagnose diseases. There are highly specific markers for enzymes active in the pancreas, red blood cells, liver, heart, brain, prostate gland and many of the endocrine glands [41]. From the time when these enzymes became comparatively easy to examine using automated techniques, they have been part of the standard blood tests that veterinarians and medical doctors are likely to need in the diagnosis and treatment/management of diseases.
Enzymes have two significant features that differentiate them from all other types of drugs. First, enzymes frequently bind and act on their targeted sites with high affinity and specificity. Second, enzymes are catalytic and convert numerous target molecules to the desired products. These two important features make enzymes specific and potent drugs that can achieve therapeutic biochemistry in the body that small molecules cannot. These features have resulted in the development of many enzyme-based drugs for a wide range of disorders [42]. Currently, numerous enzymes are used as therapeutic agents, owing to the following features:
High specificity to their substrates. Proficient in producing the desired effect without provoking any side effects. Water soluble. Extremely effective in a biological environment.Enzymes as therapeutic agents also have some serious disadvantages which restrict their application. Their bulky structure, due to their large molecular weight, excludes them from the intracellular domain. Owing to their high proteinaceous nature they are highly antigenic and are rapidly cleared from blood plasma. Extensive purification from pyrogens and toxins is essential for parenteral enzymes, which increases the cost. Table 1.9 lists some therapeutically important enzymes.
Table 1.9. Therapeutically important enzymes.
| Enzyme preparation | Source | Therapeutic application |
|---|---|---|
| Aspargenase | Escherichia coli, guinea pig serum | Cytotoxic agents |
| Bromelain | Ananas comosus | Inflammation, edema |
| Chymotrypsin | Bovine pancreas | Inflammation edema ophthalmology and upper respiratory tract diseases |
| Deoxyribonuclease (DNA hydrolysis) | Bovine pancreas | Reduces viscosity of pulmonary secretions |
| Dextranase (dextran hydrolysis) | Penicillium funiculosum | Dental plaque restriction |
| Diastase (starch hydrolysis) | Malt | Amylaceous dyspepsia |
| Galactosidase (lactose hydrolysis) | Aspergillus niger | Inherited β-galactosidase deficiency |
| Hyaluronidase (mucopolysaccharide hydrolysis) | Animal testes | Increase absorption rate, increase effectiveness of local anesthetics |
| Pancreatin | Animal pancreas | Pancreatitis |
| Papain (protease) | Carica papaya | Dyspepsia and gastritis |
| Penicillinase | Bacillus cereus | Penicillin allergy |
| Plasmin (protease) | Plasminogen | Thrombotic disorders anticoagulation |
| Streptodornase (DNAase) | Streptococci | Depolymerization of DNA in purulent exudates |
| Streptokinase (protease) | Streptococci | Thromboemolic diseases |
| Tissue plasminogen activator (protease) | Recombinant DNA technology | Thromboeniolic diseases |
| Trypsin (protease) | Animal pancreas | Cleaning necrotic tissue |
| Urokinase (protease) | Human urine | Thromboemolic diseases |
In traditional medicine, proteolytic enzymes derived from plant extracts have been used for a long time In addition to proteolytic enzymes from natural resources such as plants, 'modern' enzyme therapy includes pancreatic enzymes. Therapeutically, the use of proteolytic enzymes is partly based on scientific reports and is partly empirical [43]. Clinical evidence of the use of proteolytic enzymes in cancer studies has typically been obtained with an enzyme preparation comprising a combination of papain, trypsin and chymotrypsin. Earlier reports proved that enzyme therapy can reduce the adverse effects caused by radiotherapy and chemotherapy. There is also a report available that, in some types of tumors, survival may be sustained. The positive effects of systemic enzyme therapy appear to be based on its anti-inflammatory potential. Nevertheless, the exact mechanism of action of systemic enzyme therapy remains unsolved. The proportion of proteinases to antiproteinases, which is regularly used as a prognostic marker in cancer studies, is likely to be influenced by the oral administration of proteolytic enzymes, most likely via induction of the synthesis of antiproteinases. In addition, there are many alterations of cytokine composition during treatment with orally administered enzymes, which might be a sign of the efficacy of enzyme therapy [44].
Proteases and their inhibitors have long been studied in several tumor systems. However, out of numerous promising serine and metalloproteinase inhibitors, not a single one is included in oncology at present. The present exploration for active antiproteolytic agents is in contrast to the traditional approach, as evidenced by John Beard, who proposed the management of advanced cancer using fresh pancreatic extracts whose antitumor activity was based on their proteolytic potential.
The enzymatic treatment of tumors is based on the idea of denying the abnormal cells their essential metabolic precursors such as amino acids, nucleic acids and folates. A number of enzymes have been examined and evidenced as antitumor agents. l -serine dehydratase, l -arginase, carboxypeptidase G (folate depletion), l -asparaginase, l -methioninase, l -phenylalanine ammonia lyase, l -glutaminase, l -tyrosinase and xanthine oxidase have been studied for their anticancer activity. Enzyme preparations such as asparaginase (amidase), bromelain (protease) and chymotrypsin (protease) have also been studied as cancer treatments (table 1.9).
l -asparaginase is the most widely investigated enzyme. It has been reported in treatment against three neoplastic diseases, acute lymphoblastic leukemia, leukemic lymphosarcoma and myeloblastic leukemia. It deprives the cancerous cells of their nutritional asparagine supply. Asparagine is essential for protein synthesis, which takes place inside the cell, and decreased protein synthesis perhaps accounts for the immunosuppression and toxic effects of asparaginase-based treatment.
The prospects of enzyme-based treatment against cancer are very bright, but the difficulties of antigenicity and short circulation time remain to be overcome.
Activation of the blood clotting mechanism during inflammation is part of the body's defense mechanism which requires therapeutic intervention. Under normal physiological conditions there is an equilibrium between blood coagulation (clotting) and fibrinolysis (the process of dissolving the clotted blood) [47]. Biocatalysts such as enzymes, ribozymes, pro-enzymes, activators and pro-activators are responsible for maintaining equilibrium between clot formation and fibrinolysis. Imbalances in the concentration of these bio-activators may disturb physiology. In the biological process of fibrogenesis, clot formation takes place due to the plasma protein (soluble fibrinogen), which is ultimately converted to insoluble fibrin by the enzyme thrombin. This process is dependent on the conversion of thrombin from prothrombin. This bio-conversion takes place after the cascade of enzymatic reactions which involved certain key biological compounds called clotting factors. A blood clot dissolving enzyme known as plasmin is present in the blood as the pro-enzyme plasminogen. During clot dissolution activators convert the plasminogen to plasmin. This biological process is well regulated by certain process such as vasoconstriction, formation of a fibrin and clot platelet aggregation [46].
As the body utilizes enzymes in conserving this key balance of homeostasis, in a similar way we can utilize enzymes to repair or restore the homeostatic balance once it is lost. Several reports have shown that one of the best approaches for treating such clinical conditions is the administration of enzymes capable of converting plasminogen to plasmin (the enzyme which dissolves the clot) via intraveneous injection. This type of treatment is called therapeutic thrombolysis or thrombolytic therapy. In this treatment, pharmacological agents are used to medically induce clot breakdown [47]. Various novel thrombolytic agents have been derived from different sources for therapeutic use, such as from bacteria (streptokinase), the venom of the Malayan pit viper (Arvin), a filamentous fungus Koji mold Aspergillus oryzae (brinase), a South American snake (reptilase) and human urine (urokinase) [47].
Current advancements in thrombolytic therapy are more focused on the treatment of occlusions (blockages) of blood vessels. These types of therapy can be considered as life-saving and emergency medicine for life-threatening conditions such as myocardial infarction and massive pulmonary embolism, which are the most common reasons for cardiac arrest. This life-saving treatment is more reliable in preventing the blockages of vessels in the lungs and heart. Artery blockage conditions such as pulmonary embolism in the lungs by the formation of a clot creates tension on the right side of the heart, resulting in shortness of breath and chest pain mainly upon breathing in. Enzyme-based thrombolysis for treating massive pulmonary embolism has been considered as an effective approach to dissolving clots in these large vessels. Since surgical removal raises the chances of new blood clot formation that can cause another pulmonary embolism at the same or a different site, it is considered a dangerous practice and thrombolytic therapy is considered the more effective treatment [47]. Nevertheless, reoccurrence of clot formation or clot re-formation is very common in patients who have undergone enzyme-based thrombolytic treatment. Researchers from various organizations (1971) determined the effectiveness of streptokinase over heparin in reducing the chances of death in acute myocardial infarction patients. Significant results were obtained during this experiment. As discussed above, re-formation of the clot is one of the major concerns in fibrinolytic therapy. Most clinicians start treatment with a high dose of fibrinolytic agents, which is reduced later on. This approach may reduce disease progression for some time, but often increases the chances of clot re-formation. Even after the dissolution of the clot it is very difficult to maintain the same physiologically balanced environment (homeostasis) at the site of damaged tissues and the chance of new clot formation at that particular location is very high. Therefore, fibrinolytic based treatment is always accompanied by anticoagulants, such as heparin [46].
Major concerns associated with streptokinase therapy are fever, a tendency for bleeding, antigenicity (as with any foreign protein) and the difficulty of determining the proper dose [47]. Post-enzymatic treatment bleeding is one of the major concerns and it is also a concern when anticoagulants are used alone. According to current research, urokinase (produced in the kidneys and obtained from human urine) is considered safer than streptokinase. For the production of urokinase, 2300 l of urine is required to yield only 29 mg of purified urokinase, thus considering the expense involved in its manufacture, its clinical utilization has been restricted. Other examples are Arvin and reptilase. Utilization of these has been restricted for several reasons, but they are still considered as potential replacements for heparin as anticoagulants. Some researchers have noticed that optimum dose plays an important role and is one of the key factors in determining re-clot formation. Thorough investigation is required to overcome any shortcomings and increase the acceptance of these enzymes in therapeutic use [47].
Enzymes play an essential role in the management of various digestive disorders, such as exocrine pancreatic insufficiency [48]. Supplementation with enzymes may also be advantageous for other conditions associated with poor digestion, such as lactose intolerance. Generally, pancreatic enzymes such as porcine and bovine have been the preferred form of supplementation for exocrine pancreatic insufficiency [48]. Utilization of microbe-derived lipase has presented promise with reports showing benefits alike to pancreatic enzymes, but with a lower dosage concentration and a broader pH range. The safety and efficacy of enzymes derived from microbial species in the treatment of conditions such as malabsorption and lactose intolerance is promising. Plant-derived enzymes, e.g. bromelain from pineapple, serve as active digestive aids in the breakdown of proteins. Synergistic properties have also been reported using a combination of animal-based enzymes and microbe-derived enzymes or bromelain. Buccal administration of pancreatin (derived from an alcoholic extract of animal pancreas) enhances the enzymatic digestion of starch and proteins in patients with pancreatic cysts and pancreatitis. Pancreatin in combination with lipase is used to treat patients with fatty stools. Hydrolytic enzymes such as papain and fungal extracts (Aspergillus niger and Aspergillus otyzae) are used to enhance absorption from the small intestine [49]. These fungal extracts comprise amylases and proteases along with cellulases, which support the breakdown of the otherwise indigestible fibers of cabbages, etc, and thus reduce dyspepsia and flatulence [50]. Currently, micro-organisms are used at a large scale for the production of therapeutic enzymes. Among various micro-organisms Saccharomyces cerevisiae, Saccharomyces fragilis, Bacillus subtilis and two Aspergillus species are considered safe by the FDA (USA) for obtaining oral β-galactosidase (from A. oryzae) which is often used by patients suffering from inherited intestinal disease lactose deficiency [51]. Children with this genetic disorder children are incapable of digesting milk lactose. Enzymatic preparations such as β-galactosidase catalyze the conversion of lactose to glucose and galactose, which are quickly absorbed by the intestine. Other enzymatic preparations, e.g. penicillinase (from B. subtilis) are often used to treat hypersensitivity reactions caused by the antibiotic penicillin [52]. This enzyme catalyzes the conversion of penicillin to penicillanic acid, which is non-immunogenic. In addition, microbial and plant hydrolases are also used to decrease inflammation and edema [53]. Thrombin, trypsin, chymotrypsin, papain, streptokinase, streptodornase and sempeptidase are under clinical trial investigation. These enzymatic preparations are administered orally and have considerable proteolytic activity in the serum. Streptodornase has also displayed pain-relieving action on systemic injection [54]. Preparations have also been used to clean dirty wounds and necrotic tissue and to remove debris from second and third degree burns.
1.10. Plants and algae enzyme systems
Plant based foods are usually consumed in their raw form [68]. This eases the main concern with animal-based enzymes by preserving the integrity of the enzymes themselves. Moreover, plant-based digestive enzymes are effective over a broad scope of pH levels. This range is usually between 3.0 and 9.0, which is highly well-matched with the human gastrointestinal environment [55–72]. Thus plant-based enzymes are compatible for supporting comprehensive digestive health. Protease, amylase, lipase and cellulose are the important enzymes and are present in plants. Protease breaks down protein that can be present in meat, fish, poultry, eggs, cheese and nuts. Amylase assists your body with the breakdown and subsequent absorption of carbohydrates and starches. Lipase aids the digestion of fat. When your diet includes lipase-rich foods, it eases the production burden on the gall bladder, liver and pancreas. Cellulase is present in many fruits and vegetables, and it breaks down food fibers, which increases their nutritional value to our bodies. The presence of cellulase in plant-based sources is important, because it is not naturally present in the human body. Fruits and vegetables are an ideal source for enzymes. They are enzyme-rich and easily consumed without needing to be cooked or processed, ultimately preserving the full functionality of the enzymes. By using plant biotechnology several enzymes can be produced from plants as well algal resources [56–72].
During algal photosynthesis various proteins and enzymes are produced which can be utilized in economic development and environment management, such as in wastewater treatment, production of fine chemicals, and biodiesel production [56–72]. Due to their potential to capture and fix carbon dioxide using solar energy, photosynthetic marine algae are considered as potential models for the production of proteins. It has been recently observed that algal chloroplasts can be transformed for the production recombinant proteins [55]. Five different classes of recombinant enzymes; xylanase, α-galactosidase, phytase, phosphate anhydrolase, and β-mannanase, D. tertiolecta or C. reinhardtii were in the plastids of D. tertiolecta or C. reinhardtii. Similar strategies should allow for recombinant protein production in many species of marine algae [55].
B, brain; E, erythrocytes; H, heart muscle; Ht, hepatobiliary tract; I, intestinal mucosa; K, kidney; L, M, skeletal muscle; Pa, pancreas; P1, placenta; Pr, prostate gland; S, saliva.